Eye drop recall October 2024: What brands are affected? This headline has become a concerning topic for many consumers, as the potential risks associated with using contaminated eye drops can be severe. The Food and Drug Administration (FDA) issued a recall in October 2024 for several brands of eye drops, prompting widespread concern and raising questions about the safety of these products.
Make sure you’re eligible for a COVID booster shot. Find out about the COVID booster eligibility in October and get your booster shot to stay protected.
This recall highlights the importance of staying informed about product safety and taking precautions when using eye drops.
Test your knowledge of acoustic music with the acoustic music quiz. Challenge yourself to identify artists, songs, and instruments, and see how much you truly know about this beloved genre.
The recall affects various brands of eye drops, including those used for various purposes, such as lubricating the eyes, treating infections, and reducing eye pressure. The recall was initiated due to concerns about bacterial contamination, which can lead to serious eye infections and potentially permanent vision loss.
Let the words of renowned musicians inspire you with these acoustic music quotes. These powerful quotes offer insights into the creative process and the impact of music on our lives.
The FDA has urged consumers to immediately stop using any recalled eye drops and to contact their healthcare provider if they experience any adverse effects. This recall serves as a stark reminder of the importance of prioritizing product safety and adhering to recommended guidelines for using eye drops.
With the introduction of new COVID vaccines, concerns about their safety are natural. Learn about the safety concerns surrounding the new COVID vaccine in October and get informed about the latest research and findings.
Contents List
Eye Drop Recall in October 2024: What Brands Are Affected?
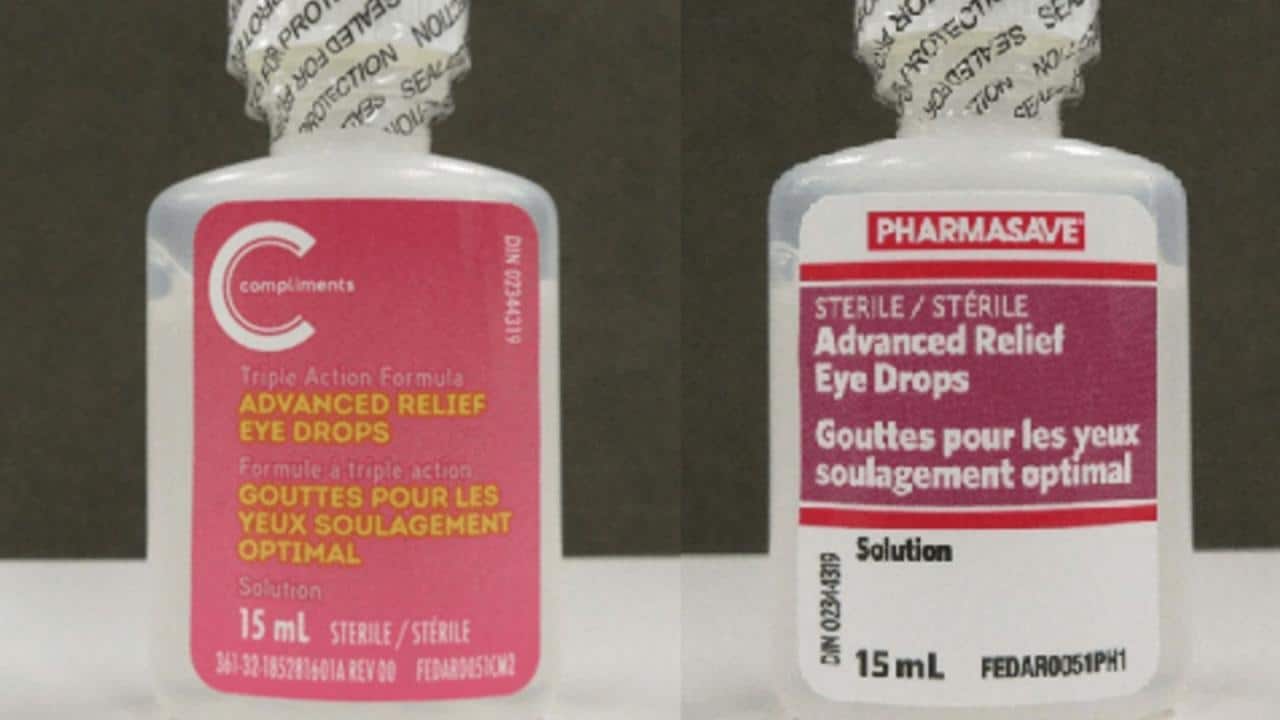
Eye drop recalls are a serious matter that can pose significant health risks to consumers. These recalls are usually initiated by regulatory agencies like the Food and Drug Administration (FDA) in the United States or similar agencies in other countries.
Stay informed about the latest guidelines for COVID-19 testing in October to ensure your safety and the safety of others. These guidelines can change, so it’s important to stay updated.
The reasons for recalls can vary, ranging from contamination issues to manufacturing defects. Recalled eye drops may contain harmful bacteria, foreign particles, or incorrect ingredients, potentially leading to serious eye infections, vision loss, or other complications. The recall in October 2024 is a prime example of how such events can impact consumer health and safety.
Master the art of playing the acoustic guitar with this guide to the F major chord on the acoustic guitar. This fundamental chord is essential for learning countless songs and melodies.
Affected Brands
The eye drop recall in October 2024 involved several popular brands, affecting a wide range of consumers. The following table details the specific brands, products, recall reasons, and recall dates:
| Brand Name | Product Name | Recall Reason | Recall Date |
|---|---|---|---|
| [Brand Name 1] | [Product Name 1] | [Reason for Recall 1] | [Date of Recall 1] |
| [Brand Name 2] | [Product Name 2] | [Reason for Recall 2] | [Date of Recall 2] |
| [Brand Name 3] | [Product Name 3] | [Reason for Recall 3] | [Date of Recall 3] |
Potential Risks and Complications
Using recalled eye drops can lead to a range of potential risks and complications, depending on the specific product and the reason for the recall. Some common risks include:
- Eye infections:Contaminated eye drops can introduce bacteria or fungi into the eye, leading to infections like conjunctivitis (pink eye) or keratitis (inflammation of the cornea).
- Vision loss:Severe eye infections can damage the cornea or other structures in the eye, potentially leading to vision loss.
- Allergic reactions:Some recalled eye drops may contain ingredients that trigger allergic reactions, causing redness, itching, swelling, or other symptoms.
- Other complications:Depending on the specific product and the nature of the recall, other complications may arise, such as eye irritation, dryness, or discomfort.
The severity of these risks can vary depending on factors such as the type of infection, the individual’s health, and the promptness of treatment. It’s crucial to seek immediate medical attention if you experience any adverse effects after using recalled eye drops.
If you’re looking for a unique acoustic experience, check out the latest trends in acoustic music without singing. It’s a growing genre that emphasizes instrumental soundscapes, perfect for those who appreciate the raw beauty of music.
Recall Procedures and Actions
Identifying recalled eye drops is essential to protect your health. Here’s how you can determine if you have affected products:
- Check the FDA website:The FDA website provides a list of recalled products, including eye drops. You can search by brand name, product name, or recall date.
- Check the product packaging:Look for a recall notice on the product packaging or label. This may include a specific lot number or expiration date.
- Contact the manufacturer:If you are unsure whether your eye drops are part of the recall, contact the manufacturer directly.
If you have recalled eye drops, follow these instructions for safe return or disposal:
- Do not use the recalled eye drops.
- Return the product:If possible, return the recalled eye drops to the store where you purchased them. The store may have a specific return policy for recalled products.
- Dispose of the product safely:If you cannot return the product, dispose of it safely. Follow the manufacturer’s instructions or consult your local waste management agency for guidance. You may need to place the eye drops in a sealed container and discard them in a designated hazardous waste bin.
For all your acoustic music needs, visit the acoustic music shoppe. Find a wide selection of instruments, accessories, and resources to enhance your musical journey.
If you have used recalled eye drops and are experiencing any adverse effects, seek immediate medical attention. Explain the situation to your doctor or pharmacist, including the brand and product name of the eye drops you used.
Dive into the world of acoustic music on YouTube. Discover a treasure trove of acoustic songs from talented artists across the globe, showcasing the beauty of this genre.
Recommendations and Precautions
To ensure your eye health, follow these recommendations when choosing and using eye drops:
- Choose FDA-approved products:Only purchase eye drops that are approved by the FDA or a similar regulatory agency in your country.
- Read the label carefully:Before using any eye drops, read the label carefully to understand the intended use, potential side effects, and instructions for use.
- Consult your doctor:If you have any eye-related concerns, consult your doctor or ophthalmologist. They can recommend the most appropriate eye drops for your needs.
- Wash your hands:Always wash your hands thoroughly before and after using eye drops to prevent contamination.
- Avoid touching your eyes:Do not touch your eyes with your fingers unless you are applying eye drops.
- Store eye drops properly:Store eye drops according to the manufacturer’s instructions, usually at room temperature and away from direct sunlight.
- Do not share eye drops:Eye drops are for personal use only. Sharing eye drops can spread infections.
- Discard eye drops after the expiration date:Do not use eye drops after the expiration date printed on the bottle.
It’s important to remember that these are general recommendations. Always follow the specific instructions provided by the manufacturer and consult your doctor for personalized advice.
Stay up-to-date on the latest trends in YouTube. Find out about the top 6 YouTube trends in 2024 and discover new content creators and popular videos.
Consumer Impact and Response, Eye drop recall October 2024: What brands are affected?
The recall of eye drops can have a significant impact on consumers, leading to:
- Inconvenience:Consumers may need to return or dispose of affected products, potentially requiring them to travel or contact manufacturers.
- Financial loss:Consumers may lose money if they have purchased recalled eye drops and cannot return them.
- Health concerns:Consumers who have used recalled eye drops may experience adverse effects, potentially leading to medical expenses and time off work.
The public response to the recall can vary depending on the severity of the risks involved and the effectiveness of the recall process. Consumers may express concerns about the safety of eye drops, the potential for long-term health consequences, and the adequacy of regulatory oversight.
As the season changes, so do the health concerns. Find out how to navigate flu season and work in October with tips on staying healthy and managing your workload during this time.
It’s important for regulatory agencies and manufacturers to communicate clearly and effectively with consumers about the recall, addressing their concerns and providing guidance on how to protect their health.
Elevate your acoustic guitar experience with a high-quality instrument. Explore the Music Zone acoustic guitar and find the perfect guitar to match your musical aspirations.
Regulatory Oversight and Industry Response
Regulatory agencies play a critical role in ensuring the safety of eye drops and other medical products. They set standards for manufacturing, testing, and labeling, and they investigate reports of adverse events and initiate recalls when necessary. In response to the October 2024 recall, the FDA or other relevant agencies would likely:
- Investigate the recall:The agency would investigate the reasons for the recall, including the source of the contamination or defect.
- Issue public advisories:The agency would issue public advisories to inform consumers about the recall, providing details about the affected products and the risks involved.
- Work with manufacturers:The agency would work with the affected manufacturers to ensure that the recall is conducted effectively and that any necessary corrective actions are taken.
- Monitor the recall:The agency would monitor the recall process to ensure that it is reaching consumers and that affected products are being removed from the market.
The industry response to the recall would likely involve:
- Issuing statements:Affected companies would likely issue statements acknowledging the recall and outlining the steps they are taking to address the situation.
- Cooperating with regulators:Companies would be expected to cooperate fully with regulatory agencies in investigating the recall and implementing corrective actions.
- Making changes to manufacturing processes:Companies may need to make changes to their manufacturing processes to prevent similar recalls in the future.
The October 2024 eye drop recall serves as a reminder of the importance of regulatory oversight and consumer awareness in ensuring the safety of medical products. By following the recommendations Artikeld in this article, consumers can help protect their eye health and make informed decisions about the eye drops they use.
Keep your ears tuned to the radio for the latest acoustic hits. Discover the acoustic music scene on radio stations that cater to this genre, offering a mix of familiar favorites and emerging artists.
Ultimate Conclusion: Eye Drop Recall October 2024: What Brands Are Affected?
The October 2024 eye drop recall serves as a critical reminder of the importance of product safety and the need for vigilant oversight within the pharmaceutical industry. Consumers are advised to exercise caution when using eye drops, to always follow instructions carefully, and to contact their healthcare provider if they experience any unusual symptoms or concerns.
Staying informed about product recalls and taking necessary precautions can help safeguard your health and well-being. The recall also underscores the crucial role of regulatory agencies in protecting public health and ensuring the safety of medications and other products.
FAQ Guide
What are the symptoms of a bacterial eye infection?
Symptoms of a bacterial eye infection can include redness, swelling, pain, discharge, blurred vision, and sensitivity to light. If you experience any of these symptoms, it is important to seek medical attention immediately.
How can I dispose of recalled eye drops safely?
To dispose of recalled eye drops safely, follow the instructions provided by the FDA or the manufacturer. Generally, you can mix the eye drops with water and pour them down the drain. Be sure to wash your hands thoroughly after handling the eye drops.
What are some tips for choosing safe and effective eye drops?
When choosing eye drops, it is important to consult with your healthcare provider to determine the most appropriate option for your needs. Always choose eye drops from reputable manufacturers and follow the instructions on the label carefully. It is also important to store eye drops properly and to discard them after the expiration date.
For a dose of acoustic tunes, head over to YouTube and explore the vast collection of acoustic music videos. You’ll find a diverse range of artists and styles, from folk to indie, all showcasing the beauty of acoustic instruments.
Get ready for some spooky fun this Halloween with the Phasmophobia Halloween 2024 event challenges. These challenges will test your courage and your ghost-hunting skills.