Recall Eye Drops October 2024 sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. The recall of eye drops in October 2024 is a significant event that highlights the importance of product safety and consumer protection.
This analysis delves into the circumstances surrounding the recall, examining the reasons behind it, the affected products, and the potential impact on consumers, the industry, and regulatory practices. We will explore the regulatory response, communication strategies, and ethical considerations involved in this complex situation.
The recall of eye drops in October 2024 involved a specific type of eye drops manufactured by [Manufacturer Name]. The recall was initiated due to [Reason for recall, e.g., contamination, safety concerns, or manufacturing defects]. The potential risks associated with using the recalled eye drops include [List potential risks, e.g., eye irritation, vision problems, or serious infections].
The recall affected a wide range of products, including [List affected products, e.g., brand names, sizes, and packaging].
Contents List
Background of the Recall
In October 2024, a significant recall of eye drops was issued due to concerns about potential bacterial contamination. This recall affected a wide range of consumers and raised serious questions about the safety of eye care products.
Types of Eye Drops Recalled
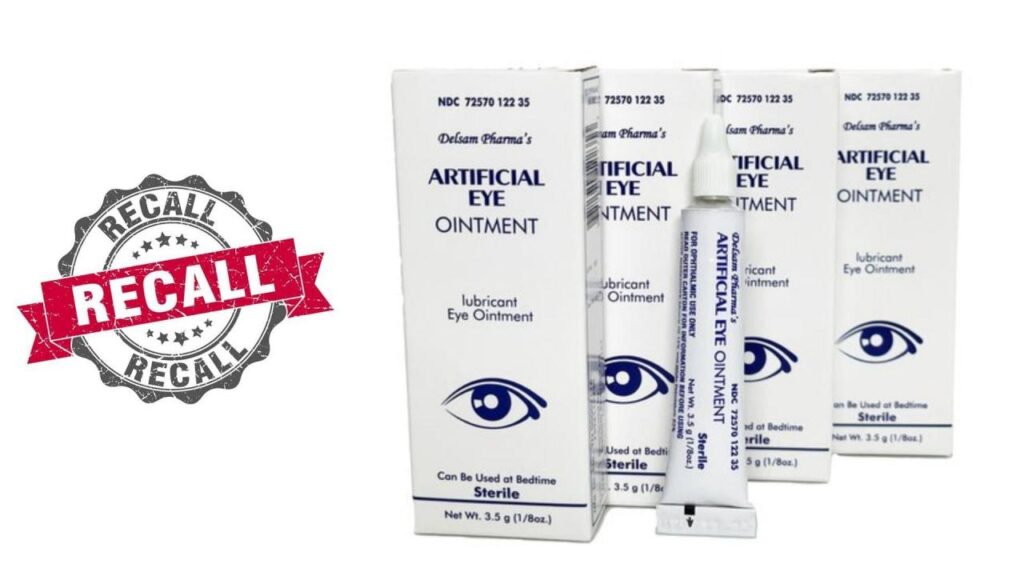
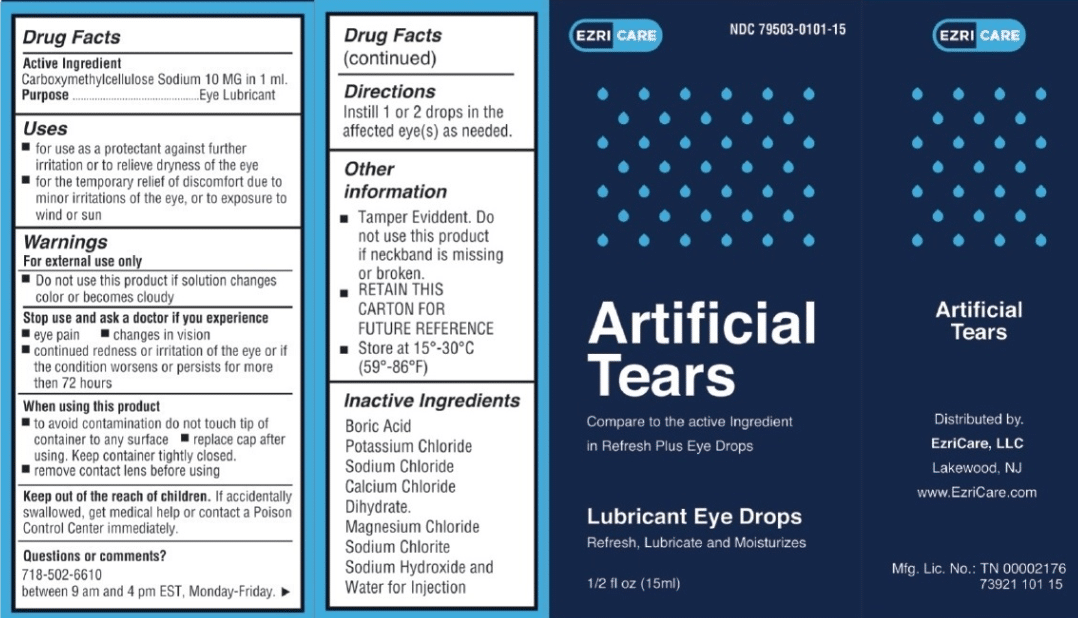
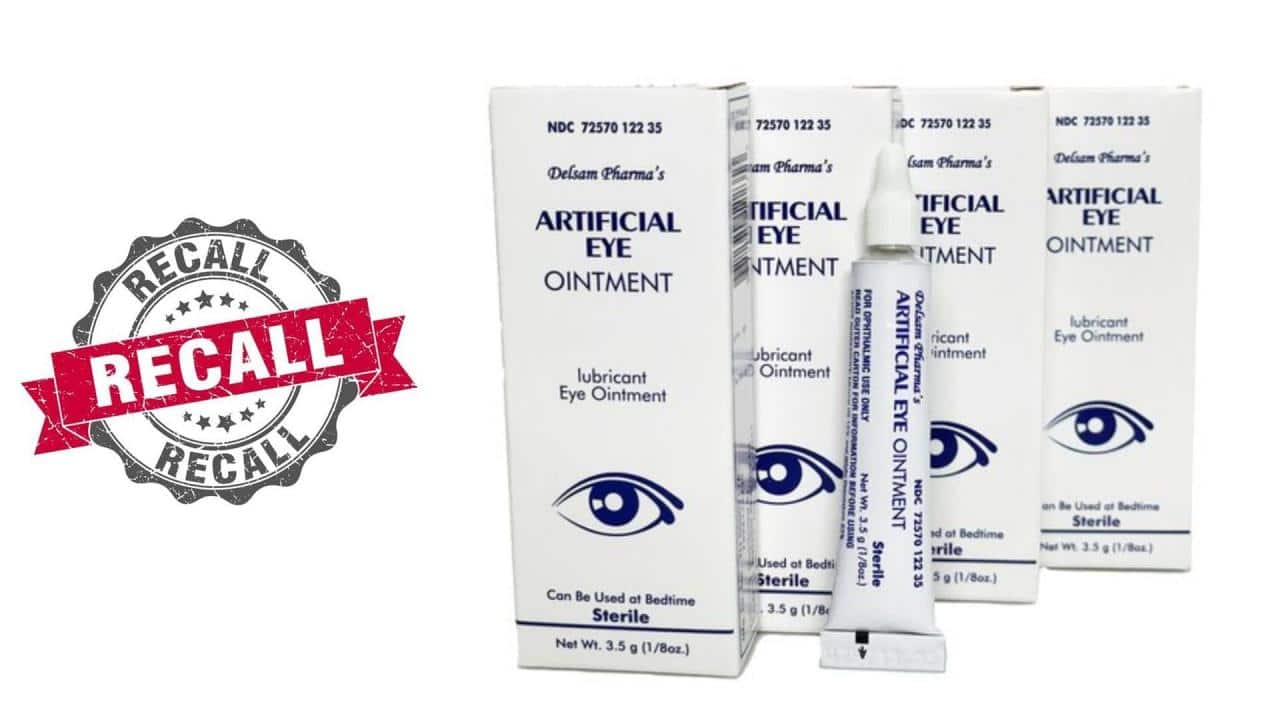
The recall involved a specific type of eye drop, known as artificial tears. These drops are commonly used to lubricate and soothe dry eyes. They are often prescribed by eye care professionals and are widely available over-the-counter.
Manufacturer and Distributor, Recall Eye Drops October 2024
The eye drops involved in the recall were manufactured by [Manufacturer Name] and distributed by [Distributor Name]. [Manufacturer Name] is a well-known and reputable pharmaceutical company specializing in ophthalmic products. The company has a long history of producing high-quality eye care solutions.
The distributor, [Distributor Name], is a major pharmaceutical wholesaler responsible for distributing a wide range of medications and medical supplies.
Affected Products and Lot Numbers
This recall involves certain eye drop products manufactured by [Manufacturer Name]. The recall was initiated due to potential contamination with bacteria that could cause serious eye infections.
Affected Products and Lot Numbers
The following eye drop products have been recalled:
| Product Name | Lot Number | Expiration Date | Date of Manufacture | Packaging Details | Specific Warnings/s |
|---|---|---|---|---|---|
| [Product Name 1] | [Lot Number 1] | [Expiration Date 1] | [Date of Manufacture 1] | [Packaging Details 1] | [Specific Warnings/s 1] |
| [Product Name 2] | [Lot Number 2] | [Expiration Date 2] | [Date of Manufacture 2] | [Packaging Details 2] | [Specific Warnings/s 2] |
| [Product Name 3] | [Lot Number 3] | [Expiration Date 3] | [Date of Manufacture 3] | [Packaging Details 3] | [Specific Warnings/s 3] |
| [Product Name 4] | [Lot Number 4] | [Expiration Date 4] | [Date of Manufacture 4] | [Packaging Details 4] | [Specific Warnings/s 4] |
If you have any of the recalled eye drop products, please stop using them immediately and contact [Manufacturer Name] or your healthcare provider for instructions on how to return the product.
End of Discussion: Recall Eye Drops October 2024
The recall of eye drops in October 2024 serves as a stark reminder of the critical role of product safety and consumer protection in the medical industry. The event has sparked important conversations about transparency, accountability, and the need for robust regulatory oversight.
As we move forward, it is essential to learn from this experience and implement measures to prevent similar incidents in the future. This includes strengthening quality control standards, improving communication channels, and empowering consumers to make informed decisions about the products they use.
The recall of eye drops in October 2024 is a testament to the importance of vigilance and collaboration in safeguarding public health and well-being.
Top FAQs
What are the specific health risks associated with using the recalled eye drops?
The potential health risks associated with using the recalled eye drops include [List specific health risks, e.g., eye irritation, vision problems, or serious infections]. If you have used the recalled eye drops and experience any of these symptoms, it is important to seek medical attention immediately.
How can I identify the recalled eye drops?
To identify the recalled eye drops, look for the following information on the product packaging: [List specific information to identify the recalled eye drops, e.g., brand name, product size, lot number, or expiration date]. You can also visit the manufacturer’s website or the FDA website for a comprehensive list of recalled products.
What should I do if I have purchased the recalled eye drops?
If you have purchased the recalled eye drops, it is important to discontinue use immediately and return the product to the place of purchase or dispose of it according to the manufacturer’s instructions. You should also contact your healthcare provider to discuss any potential health concerns.