Recalled Eye Drops October 2024: A recent recall of eye drops in October 2024 has raised significant concerns about product safety and the potential health risks associated with contaminated eye care products. This event has prompted widespread investigations into the manufacturing processes, distribution channels, and regulatory oversight of eye drop products.
The recall has impacted numerous consumers, leading to a surge in inquiries about potential health risks, treatment options, and the long-term consequences of using the affected eye drops. This report aims to provide a comprehensive overview of the situation, including details about the recalled products, affected consumers, health risks, regulatory responses, and the broader implications for the eye care industry and public health.
The recall has highlighted the importance of rigorous quality control measures, transparent communication, and consumer awareness in ensuring the safety of eye care products. It has also sparked discussions about the need for enhanced regulatory oversight and the development of more effective strategies to prevent future recalls.
This event serves as a crucial reminder of the potential consequences of product contamination and the critical role of vigilance in safeguarding consumer health.
Contents List
- 1 Comparison to Previous Eye Drop Recalls
- 2 10. Public Health Perspective: Recalled Eye Drops October 2024
- 3 11. Legal and Ethical Considerations
- 4 Alternative Eye Care Products
- 5 Eye Care Safety Tips
- 6 15. Long-Term Effects and Monitoring
- 7 Wrap-Up
- 8 Commonly Asked Questions
Comparison to Previous Eye Drop Recalls
The October 2024 recall of eye drops, like many before it, underscores the importance of vigilance in the pharmaceutical industry. Recalls, while regrettable, play a crucial role in safeguarding public health. Analyzing trends and patterns in past recalls can provide valuable insights for prevention and improvement in the future.
Reasons for Recalls
Recalls of eye drops can stem from a variety of factors, including contamination, manufacturing defects, and labeling errors. Examining the reasons behind previous recalls can highlight recurring issues and potential areas for greater scrutiny.
- Microbial Contamination:A significant portion of eye drop recalls have been attributed to microbial contamination. This can occur during manufacturing, packaging, or even after the product has been opened by the consumer. The presence of bacteria, fungi, or other microorganisms can lead to serious eye infections.
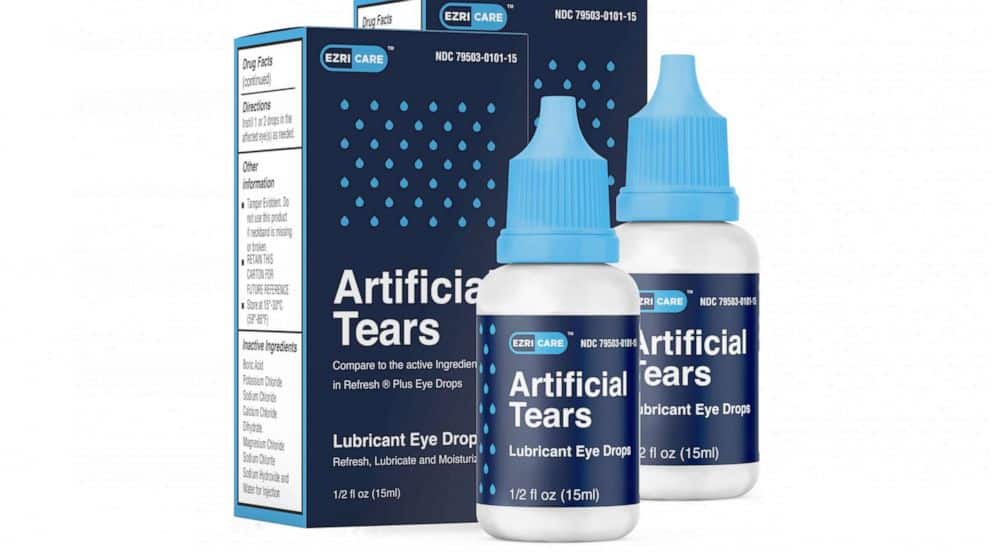
Examples of such recalls include those involving EzriCare Artificial Tears and Bausch + Lomb Biotrue One-day Disposable Contact Lenses, both of which were linked to outbreaks of drug-resistant bacteria.
- Manufacturing Defects:Defects in the manufacturing process can also lead to recalls. These defects might involve issues with the product’s formulation, packaging, or sterility. For instance, a recall of Alcon’s Systane Ultra eye drops in 2023 was due to a manufacturing issue that resulted in the presence of particulate matter in the bottles.
- Labeling Errors:Inaccurate or misleading labeling can also necessitate a recall. This might involve incorrect dosage instructions, missing warnings, or inaccurate ingredient lists. A recall of Refresh Tears eye drops in 2022 was initiated due to a labeling error that omitted a critical warning about potential side effects.
Scope of Recalls
The scope of an eye drop recall can vary widely, ranging from limited regional recalls to nationwide or even global recalls. The extent of a recall is often influenced by the severity of the potential risk and the distribution of the affected product.
- Limited Recalls:Recalls may be limited to specific batches or regions if the risk is considered low or if the affected product has a limited distribution. For example, a recall of a certain batch of artificial tears might be confined to a particular state if the contamination was detected during routine testing.
- Nationwide Recalls:Recalls can be expanded to a nationwide level if the risk is considered more significant or if the affected product is widely distributed. The recall of EzriCare Artificial Tears, for instance, was nationwide due to the potential for serious bacterial infections.
- Global Recalls:In some cases, recalls can extend beyond national borders, particularly if the affected product is sold internationally. The recall of Bausch + Lomb Biotrue One-day Disposable Contact Lenses, linked to a multi-state outbreak of a drug-resistant bacteria, was expanded globally to encompass all affected products.
Consequences of Recalls
Eye drop recalls can have significant consequences for both consumers and manufacturers. These consequences can include health risks, financial losses, and reputational damage.
- Health Risks:The most serious consequence of an eye drop recall is the potential for harm to consumers. Contaminated or defective eye drops can cause eye infections, vision loss, and other serious health problems. In some cases, these health risks can be long-term or even permanent.
- Financial Losses:Recalls can be costly for manufacturers, involving expenses for product retrieval, disposal, and communication with consumers. Additionally, companies may face lawsuits and fines if their products are found to be defective or harmful.
- Reputational Damage:Recalls can damage a manufacturer’s reputation, leading to decreased consumer trust and sales. This damage can be difficult to repair, even if the company takes steps to address the issue.
Trends and Patterns
Analyzing trends and patterns in eye drop recalls over time can provide valuable insights into the evolving landscape of this industry. Several key trends have emerged in recent years.
- Increased Frequency:There has been a noticeable increase in the frequency of eye drop recalls in recent years. This trend can be attributed to a number of factors, including increased scrutiny by regulatory agencies, heightened awareness of potential risks, and the growing complexity of eye drop formulations.
- Microbial Contamination Concerns:Microbial contamination remains a significant concern in the eye drop industry. This is partly due to the challenges associated with maintaining sterility throughout the manufacturing and packaging processes. The rise of multi-dose eye drop bottles, which are more susceptible to contamination, has also contributed to this trend.
- Focus on Safety:The pharmaceutical industry has placed an increasing emphasis on safety in recent years. This focus has led to more stringent testing procedures, improved manufacturing practices, and greater transparency regarding potential risks.
10. Public Health Perspective: Recalled Eye Drops October 2024
The recall of eye drops presents a significant public health concern, potentially impacting a wide range of individuals and posing risks to their eye health. It is crucial to understand the potential health implications, the vulnerability of specific populations, and the effectiveness of public health communication strategies in mitigating these risks.
Specific Health Risks, Recalled Eye Drops October 2024
The potential health risks associated with using the recalled eye drops depend on the specific contaminants or manufacturing issues identified. These risks can range from mild irritation to severe infections, potentially leading to permanent vision loss.
- Bacterial Infections:Contaminated eye drops can introduce bacteria into the eye, leading to infections like conjunctivitis (pink eye), keratitis (inflammation of the cornea), and endophthalmitis (infection inside the eye). These infections can cause pain, redness, swelling, discharge, and blurred vision. In severe cases, endophthalmitis can lead to vision loss or even blindness.
- Fungal Infections:Some recalled eye drops may be contaminated with fungi, which can cause fungal keratitis, a serious eye infection that can lead to corneal scarring and vision loss.
- Allergic Reactions:The recalled eye drops may contain ingredients that cause allergic reactions, resulting in itching, redness, swelling, and tearing.
- Other Potential Risks:Depending on the specific recall, other risks may include chemical burns, corneal abrasions, or damage to the eye’s surface.
Vulnerable Populations
Certain populations are particularly vulnerable to the effects of contaminated eye drops:
- Individuals with Pre-existing Eye Conditions:People with pre-existing eye conditions, such as dry eye, glaucoma, or corneal disease, are more susceptible to developing complications from contaminated eye drops. Their compromised eye health may make them more vulnerable to infections and other complications.
- Pregnant Women:Pregnant women should avoid using any eye drops unless specifically prescribed by their doctor. The potential risks associated with contaminated eye drops may pose a threat to both the mother and the developing fetus.
- Children:Children are more susceptible to eye infections and may have difficulty following safety instructions. They should be closely monitored and supervised when using eye drops.
Long-Term Consequences
The long-term consequences of using recalled eye drops can vary depending on the severity of the infection or complication. In some cases, the effects may be temporary, while others can lead to permanent vision loss.
- Vision Loss:Severe infections, such as endophthalmitis, can lead to permanent vision loss if not treated promptly. Corneal scarring from fungal keratitis can also result in impaired vision.
- Chronic Eye Problems:Repeated or persistent infections can lead to chronic eye problems, such as dry eye, glaucoma, or cataracts.
- Psychological Impact:Vision loss or chronic eye problems can have a significant psychological impact, affecting self-esteem, quality of life, and independence.
11. Legal and Ethical Considerations
The recall of eye drops raises significant legal and ethical considerations, impacting the manufacturer, distributors, healthcare providers, and most importantly, the consumers who relied on these products for their eye health. This section explores the potential legal ramifications of the recall, examines the regulatory scrutiny the manufacturer will face, and delves into the ethical dilemmas that arise from this situation.
Potential Lawsuits
A class-action lawsuit against the eye drop manufacturer is a likely scenario following the recall. Plaintiffs, representing affected consumers, would likely argue that the manufacturer was negligent in producing and distributing contaminated eye drops.
Plaintiffs’ Arguments
- The manufacturer failed to implement adequate quality control measures, leading to the contamination of the eye drops.
- The manufacturer failed to warn consumers about the potential risks associated with using the contaminated eye drops.
- The manufacturer’s actions caused consumers to suffer injuries, including eye infections, vision loss, and other health complications.
Defendants’ Arguments
- The manufacturer may argue that the contamination was an isolated incident and that they took reasonable steps to ensure the safety of their products.
- The manufacturer may argue that consumers failed to follow proper instructions for using the eye drops, contributing to their injuries.
- The manufacturer may argue that the plaintiffs’ injuries were not directly caused by the contaminated eye drops but by other factors.
Potential Damages
- Medical expenses for treating eye infections and other health complications.
- Pain and suffering, including emotional distress and loss of enjoyment of life.
- Loss of wages if the injuries prevented consumers from working.
- Punitive damages, which are intended to punish the manufacturer for their wrongdoing and deter future misconduct.
| Arguments | Plaintiffs | Defendants |
|---|---|---|
| Negligence | Manufacturer failed to implement adequate quality control. | Contamination was an isolated incident. Reasonable steps were taken to ensure safety. |
| Failure to Warn | Manufacturer failed to warn consumers of potential risks. | Consumers failed to follow proper instructions. |
| Causation | Injuries were directly caused by contaminated eye drops. | Injuries were not caused by contaminated eye drops but by other factors. |
Regulatory Investigations
The FDA would likely initiate an investigation into the eye drop recall, focusing on the manufacturer’s manufacturing processes, quality control measures, and communication with consumers.
FDA’s Focus
- The FDA would investigate the manufacturer’s manufacturing facility, examining their production processes, equipment, and sanitation practices.
- The FDA would review the manufacturer’s quality control records, including testing results, batch records, and complaint logs.
- The FDA would assess the manufacturer’s communication with consumers, including their recall notification process and their response to consumer inquiries.
Regulatory Violations
- Failure to comply with Good Manufacturing Practices (GMPs).
- Failure to adequately test and control the quality of their products.
- Failure to promptly report adverse events and recall contaminated products.
- Failure to provide accurate and timely information to consumers about the recall.
Potential Consequences
- Fines and penalties.
- Product bans or restrictions.
- Criminal charges against individuals responsible for the contamination.
- Reputational damage and loss of consumer trust.
FDA Investigation Steps
- Initial assessment of the recall and its potential public health impact.
- Collection of information from the manufacturer, distributors, and healthcare providers.
- Inspection of the manufacturer’s facility and review of their production records.
- Laboratory analysis of the recalled eye drops to confirm the source of contamination.
- Issuance of warning letters or other regulatory actions to address violations.
- Public communication about the recall and the FDA’s investigation.
Manufacturer Responsibilities
The manufacturer is ultimately responsible for ensuring the safety of their products.
Steps to Prevent the Recall
- Implement robust quality control measures to prevent contamination during production.
- Conduct rigorous testing of their products to ensure they meet safety standards.
- Maintain accurate records of their manufacturing processes and quality control results.
- Establish a system for promptly reporting adverse events and taking corrective actions.
- Develop a clear and effective communication plan for notifying consumers of product recalls.
Legal and Ethical Obligations
- Manufacturers have a legal obligation to produce safe products and to warn consumers about potential risks.
- Manufacturers have an ethical obligation to prioritize consumer safety and to act with integrity and transparency.
Communication with Consumers
- The manufacturer should have issued a clear and concise recall notice, providing detailed information about the contaminated eye drops.
- The manufacturer should have provided instructions on how to return the contaminated eye drops and how to obtain replacement products.
- The manufacturer should have responded promptly to consumer inquiries and concerns.
Flowchart of Manufacturer Responsibilities for Product Safety
- Step 1:Develop and implement robust quality control procedures.
- Step 2:Conduct thorough product testing to ensure safety and efficacy.
- Step 3:Maintain accurate records of production processes and quality control results.
- Step 4:Establish a system for reporting adverse events and taking corrective actions.
- Step 5:Develop a clear and effective communication plan for notifying consumers of product recalls.
- Step 6:Respond promptly and transparently to consumer inquiries and concerns.
Distributor Responsibilities
Distributors play a crucial role in the supply chain, and they also have legal and ethical obligations to ensure the safety of the products they distribute.
Legal and Ethical Obligations
- Distributors have a legal obligation to ensure that the products they distribute meet safety standards.
- Distributors have an ethical obligation to prioritize consumer safety and to act with integrity and transparency.
Steps to Ensure Product Safety
- Verify the authenticity and safety of the products they receive from manufacturers.
- Maintain accurate records of their inventory and distribution activities.
- Cooperate with manufacturers and regulatory agencies in responding to product recalls.
- Inform their customers about product recalls and provide instructions on how to return recalled products.
Potential Actions to Mitigate Risks
- Immediately stop distributing recalled eye drops.
- Contact their customers who may have received the recalled products.
- Cooperate with the manufacturer in collecting and returning the recalled products.
- Provide information to customers about how to obtain replacement products.
Healthcare Provider Responsibilities
Healthcare providers play a vital role in prescribing and administering eye drops, and they have a responsibility to ensure the safety of their patients.
Legal and Ethical Obligations
- Healthcare providers have a legal obligation to prescribe and administer medications safely and effectively.
- Healthcare providers have an ethical obligation to prioritize patient safety and to act with integrity and transparency.
Response to the Recall
- Healthcare providers should immediately stop prescribing and administering the recalled eye drops.
- Healthcare providers should contact their patients who may have received the recalled eye drops.
- Healthcare providers should advise their patients about the potential risks of using the recalled eye drops.
- Healthcare providers should monitor their patients for any signs of adverse reactions to the recalled eye drops.
Steps to Ensure Patient Safety
- Stay informed about product recalls and other safety alerts.
- Verify the authenticity and safety of medications before prescribing or administering them.
- Communicate effectively with patients about the risks and benefits of medications.
- Monitor patients for any signs of adverse reactions to medications.
Checklist for Healthcare Providers Responding to a Product Recall
- Stop prescribing and administering the recalled product.
- Contact patients who may have received the recalled product.
- Advise patients about the potential risks of using the recalled product.
- Monitor patients for any signs of adverse reactions.
- Report any adverse reactions to the FDA and the manufacturer.
- Cooperate with regulatory agencies in investigating the recall.
Ethical Considerations
The eye drop recall raises significant ethical questions about consumer protection, corporate responsibility, and the importance of transparency in the pharmaceutical industry.
Ethical Principles at Stake
- Beneficence:The manufacturer has a moral obligation to act in the best interests of consumers and to avoid causing harm.
- Non-maleficence:The manufacturer has a moral obligation to avoid harming consumers through their products and practices.
- Justice:The manufacturer has a moral obligation to treat consumers fairly and equitably, regardless of their socioeconomic status or other factors.
- Autonomy:Consumers have a right to make informed decisions about their health and to have access to accurate information about the products they use.
Manufacturer’s Actions and Ethical Principles
- The manufacturer’s failure to implement adequate quality control measures and to promptly recall contaminated eye drops violates the ethical principles of beneficence, non-maleficence, and justice.
- The manufacturer’s failure to communicate transparently with consumers about the recall violates the ethical principle of autonomy.
Ethical Implications for the Industry
- The recall highlights the importance of prioritizing consumer safety and transparency in the pharmaceutical industry.
- The recall underscores the need for robust regulatory oversight and enforcement to ensure the safety of medications.
- The recall raises questions about the ethical responsibilities of manufacturers, distributors, and healthcare providers in protecting consumers.
“The ethical imperative in medicine is to do no harm, to promote the good of the patient, and to treat all patients fairly and equitably.”
The Hippocratic Oath
Alternative Eye Care Products
The recall of certain eye drops has raised concerns about the safety and effectiveness of other eye care products. It is important to understand the alternatives available and how they compare to the recalled products.
Alternative Eye Care Products
Many eye care products are available that are not affected by the recall. These products are designed to address various eye conditions, including dry eyes, allergies, and infections.
Types of Alternative Eye Care Products
- Artificial Tears:These over-the-counter (OTC) products are used to lubricate the eyes and relieve dryness. Popular brands include Systane, Refresh, and Tears Naturale. Artificial tears are generally safe and effective for most people.
- Antihistamines:These eye drops can help relieve the symptoms of allergic conjunctivitis, such as itching, redness, and watery eyes. Common antihistamine eye drops include Zaditor and Alaway.
- Antibiotics:These eye drops are prescribed by a doctor to treat bacterial infections. Examples include Tobramycin and Ciprofloxacin.
- Steroids:These eye drops are used to reduce inflammation and are often prescribed for conditions like uveitis. Examples include Prednisolone and Loteprednol.
Effectiveness and Safety of Alternative Eye Care Products
The effectiveness and safety of alternative eye care products depend on the specific product and the individual’s needs. It is important to consult with an eye doctor to determine the best product for your condition.
Comparison to Recalled Eye Drops
Alternative eye care products are generally safe and effective when used as directed. However, it is important to note that the recalled eye drops were contaminated with bacteria, which can cause serious infections.
Guidance on Selecting Safe and Effective Eye Care Products
- Consult with an eye doctor:Discuss your symptoms and eye care needs with a qualified eye doctor to receive personalized recommendations.
- Read product labels carefully:Check the ingredients, expiration date, and usage instructions before using any eye care product.
- Choose products from reputable brands:Look for products from well-established companies with a track record of safety and effectiveness.
- Store eye drops properly:Keep eye drops in a cool, dry place and discard them after the expiration date.
- Avoid sharing eye drops:Sharing eye drops can spread infections.
Eye Care Safety Tips
Maintaining good eye health is essential for overall well-being. Following proper hygiene practices and using eye care products safely can significantly reduce the risk of eye infections and other complications.
Importance of Eye Hygiene
Maintaining good eye hygiene is crucial to prevent infections and maintain healthy eyes.
- Wash your hands frequently with soap and water, especially before touching your eyes.
- Avoid touching your eyes with dirty hands or objects.
- Use a clean, soft cloth or tissue to wipe away any discharge or debris from your eyes.
- Do not share eye makeup, contact lenses, or eye drops with others.
- Replace contact lenses as recommended by your eye doctor and follow proper cleaning and storage instructions.
Safe Use of Eye Care Products
Using eye care products safely is crucial for preventing eye irritation and infections.
- Always read and follow the instructions on the product label.
- Do not use expired eye drops or other eye care products.
- Store eye drops in a cool, dry place, away from direct sunlight.
- Avoid using eye drops that are not prescribed by a doctor.
- If you experience any eye irritation or discomfort after using an eye care product, discontinue use and consult a healthcare professional.
Consulting a Healthcare Professional
It is essential to consult a healthcare professional for any eye-related concerns.
- If you experience sudden vision changes, eye pain, redness, or discharge, seek immediate medical attention.
- Schedule regular eye exams, even if you do not have any symptoms, to monitor your eye health.
- Discuss any eye care products you are using with your doctor to ensure they are safe and appropriate for your needs.
Reliable Information Sources
There are several reliable sources for obtaining information about eye care products and practices.
- The American Academy of Ophthalmology (AAO): [https://www.aao.org/](https://www.aao.org/)
- The American Optometric Association (AOA): [https://www.aoa.org/](https://www.aoa.org/)
- The National Eye Institute (NEI): [https://www.nei.nih.gov/](https://www.nei.nih.gov/)
15. Long-Term Effects and Monitoring
It’s important to understand that even if you’re not experiencing any symptoms right now, there’s still a chance that using the recalled eye drops could have long-term effects. These effects may not be immediately noticeable, but they could develop over time.While we don’t have all the answers yet, we want to be transparent and proactive in addressing any potential concerns.
Potential Long-Term Effects
While we are still learning about the long-term effects of these recalled eye drops, it’s important to be aware of the potential risks. Some individuals may experience:
- Changes in vision:This could include blurred vision, double vision, or even a decrease in your overall vision clarity.
- Eye irritation and discomfort:You might experience ongoing redness, itching, or a burning sensation in your eyes.
- Eye infections:The contaminated eye drops could increase your risk of developing an eye infection.
- Other eye problems:In rare cases, there’s a possibility of more serious eye conditions developing, such as glaucoma or corneal damage.
It’s important to remember that these are potential risks, and not everyone who used the recalled eye drops will experience these effects. However, it’s essential to be vigilant and monitor your health closely.
Symptoms to Monitor
To help you stay informed, here’s a table outlining key symptoms to monitor, both immediately and long-term, after using the recalled eye drops:
| Symptom | Severity | When to Seek Medical Attention |
|---|---|---|
| Changes in vision (blurred, double vision, decreased clarity) | Mild to Severe | Immediately, especially if sudden or significant changes occur |
| Eye irritation (redness, itching, burning) | Mild to Severe | If symptoms persist or worsen, or if accompanied by other concerning symptoms |
| Eye pain or discomfort | Mild to Severe | Immediately, especially if severe or accompanied by other symptoms |
| Increased sensitivity to light | Mild to Severe | If accompanied by other symptoms, such as vision changes or pain |
| Discharge or crusting around the eyes | Mild to Severe | If accompanied by other symptoms, such as redness or pain |
| Headache or nausea | Mild to Severe | If accompanied by other symptoms, such as vision changes or eye pain |
Social Media Message
We urge everyone who has used the recalled eye drops to be vigilant about their health. Monitor for any unusual symptoms, such as changes in vision, eye irritation, or pain. If you experience any concerns, please contact your healthcare provider immediately.
Your health is our priority.
Frequently Asked Questions
Is it possible that I could experience permanent damage from using the recalled eye drops?
While the possibility of permanent damage exists, it’s important to remember that not everyone who used the recalled eye drops will experience this. The extent of potential damage can vary depending on factors such as the duration of use and individual susceptibility.
What is the likelihood of developing complications from using the recalled eye drops?
The likelihood of developing complications varies from person to person. Some individuals may experience minor and temporary effects, while others may face more significant and lasting consequences. It’s crucial to be aware of the potential risks and seek medical attention if you experience any concerning symptoms.
How important is it to follow up with my healthcare provider after using the recalled eye drops?
Following up with your healthcare provider is essential to ensure your health and well-being. They can monitor your condition, address any concerns, and provide personalized guidance based on your individual situation.
Wrap-Up
The October 2024 eye drop recall serves as a stark reminder of the importance of product safety and the need for robust regulatory oversight in the pharmaceutical industry. The event has far-reaching implications for consumers, healthcare professionals, and the industry itself, prompting calls for enhanced quality control measures, improved communication strategies, and greater transparency in the supply chain.
As we move forward, it is essential to learn from this experience and implement measures to prevent similar incidents from occurring in the future. This includes fostering a culture of vigilance, prioritizing consumer safety, and ensuring that all stakeholders are held accountable for maintaining the integrity and quality of eye care products.
Commonly Asked Questions
What are the specific eye drops that were recalled in October 2024?
The recall involved several brands and types of eye drops. A comprehensive list of the recalled products, including brand names, product types, and lot numbers, can be found on the official websites of the manufacturers and regulatory agencies. It is crucial to consult these resources for accurate and up-to-date information.
How can I tell if my eye drops are part of the recall?
To determine if your eye drops are part of the recall, carefully check the product packaging for the brand name, product type, and lot number. Compare this information to the lists provided by the manufacturers and regulatory agencies. If you have any doubts, it is always best to contact your healthcare provider or a pharmacist for guidance.
What should I do if I have used the recalled eye drops?
If you have used the recalled eye drops, it is essential to discontinue use immediately and consult your healthcare provider. They can assess your situation, advise on any necessary follow-up care, and monitor your health for any potential complications.
It is crucial to be vigilant about your health and seek medical attention if you experience any unusual symptoms.
What are the long-term health risks associated with using the recalled eye drops?
The long-term health risks associated with using the recalled eye drops depend on the specific contaminants present and the individual’s sensitivity. Some potential long-term consequences could include vision impairment, eye infections, and other serious complications. It is essential to consult your healthcare provider for a personalized assessment and guidance based on your specific situation.
Are there any alternative eye drops available?
Yes, there are alternative eye drops available that are not affected by the recall. It is important to consult your healthcare provider to discuss appropriate alternatives based on your individual needs and eye condition. They can provide recommendations for safe and effective eye care products.